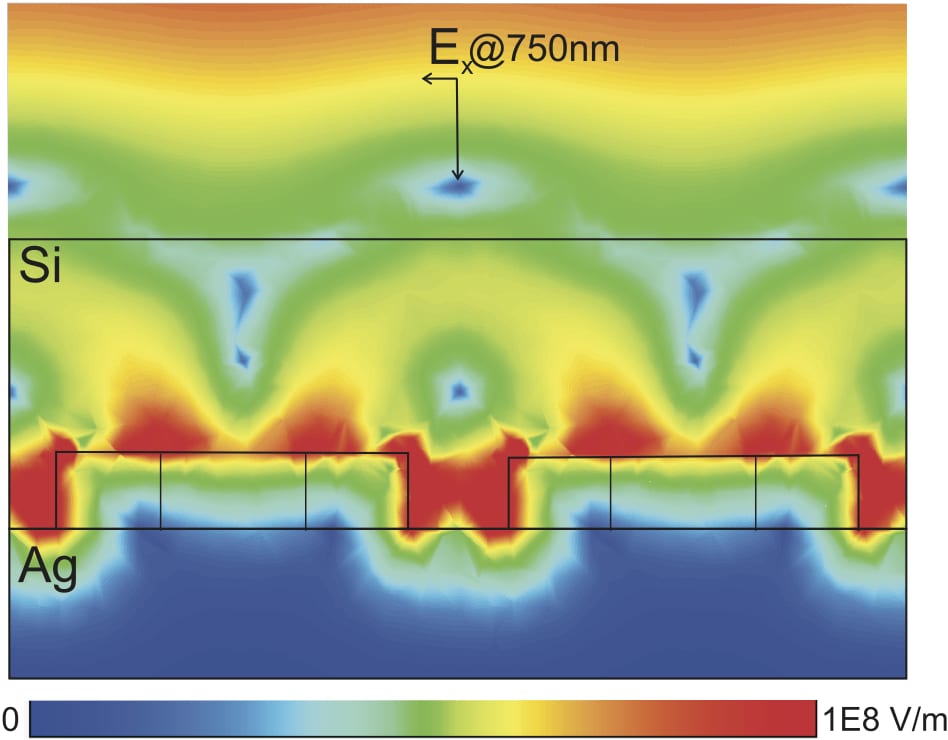
Plasmonics and Nanophotonics Noble metal nanostructures support surface plasmon excitations that result from the collective oscillation of loosely bound electrons. At the resonant frequencies, the metal is highly polarizable and obtains large field-induced polarizations that radiate. Plasmon excitation can generate surface fields that are many orders of magnitude larger than the electromagnetic field applied initially. We are using methods based on classical electrodynamics to investigate metal nanoparticles and complex nanoscale architectures for various applications. Current projects include studies of nanoparticle optical properties, energy transfer in strongly coupled nanosystems, and molecular properties in close proximity to metallic surfaces.
Noble metal nanostructures support surface plasmon excitations that result from the collective oscillation of loosely bound electrons. At the resonant frequencies, the metal is highly polarizable and obtains large field-induced polarizations that radiate. Plasmon excitation can generate surface fields that are many orders of magnitude larger than the electromagnetic field applied initially. We are using methods based on classical electrodynamics to investigate metal nanoparticles and complex nanoscale architectures for various applications. Current projects include studies of nanoparticle optical properties, energy transfer in strongly coupled nanosystems, and molecular properties in close proximity to metallic surfaces.
Low Dimensional Nanomaterials The properties of nanomaterials are often strikingly different than both the molecular level and macroscopic regime. Reducing dimensionality or system size from that of bulk can affect the material properties in fascinating and unusual ways. We are using various electronic structure methods to examine the electronic and optical properties of nanomaterials. Examples of targeted systems include semiconductor quantum dots, metal nanoparticles, and 2D materials. We utilize both molecular quantum chemistry approaches with localized basis sets as well as plane wave density functional theory to understand bonding, structure, and reactivity. The contour plot shows the electronic density of a nitrogen-doped graphene sheet. There is a localization of charge at the lattice site where nitrogen is substituted for carbon.
The properties of nanomaterials are often strikingly different than both the molecular level and macroscopic regime. Reducing dimensionality or system size from that of bulk can affect the material properties in fascinating and unusual ways. We are using various electronic structure methods to examine the electronic and optical properties of nanomaterials. Examples of targeted systems include semiconductor quantum dots, metal nanoparticles, and 2D materials. We utilize both molecular quantum chemistry approaches with localized basis sets as well as plane wave density functional theory to understand bonding, structure, and reactivity. The contour plot shows the electronic density of a nitrogen-doped graphene sheet. There is a localization of charge at the lattice site where nitrogen is substituted for carbon.
Photovoltaics and Electrical Energy Storage
Solar energy is an attractive alternative to combustion fuels; however, photovoltaic devices are still plagued by high prices and low efficiencies. Moreover, energy generated from renewable sources often suffers from a disconnect between energy production and consumer usage, highlighting an additional need for cost effective and efficient electrical energy storage. Our group is investigating the properties of unique nano-architectures for use in photovoltaic devices. The interplay between plasmonic components, semiconducting surfaces, and donor-pi-acceptor molecules is of particular interest. We are also investigating how energy is stored in supercapacitors and batteries to determine the link between interfacial chemistry and storage properties. We want to understand the processes occurring at the electrode/electrolyte interface on a molecular level to achieve higher energy and power densities in these devices. Currently, the focus is on aqueous electrolytes interacting with porous carbon electrodes to determine how confinement and hydration affect ion diffusivities, structured layer formation, and capacitance. Future work will involve investigating organic solutions and ionic liquids as electrolytes, and utilizing new electrode materials to further enhance electrical energy storage capabilities.
Photocatalysis and Solar Fuels
Photocatalysts use solar photons to drive surface redox reactions that can create chemical fuels. The energy of sunlight is effectively harnessed by the photocatalyst and stored in chemical bonds that can be extracted later as needed. The material absorbs photons with an energy greater than the band gap, which promotes electrons to the conduction band and leaves behind holes in the valence band. Charge carriers migrate to interfaces and can participate in redox chemistry with adsorbed species. Negati vely-charged electrons will reduce acceptors, and positive holes can oxidize donors if certain conditions are met. Specifically, the conduction band potential should be more negative, and valance band potential more positive, than the electrochemical potentials of desired reactions. An alternate – and less attractive outcome – is charge carrier recombination that generates heat and emission. Our group is modeling photocatalyst heterojunctions composed of metal-free 2D materials as alternatives to titania-based systems.
vely-charged electrons will reduce acceptors, and positive holes can oxidize donors if certain conditions are met. Specifically, the conduction band potential should be more negative, and valance band potential more positive, than the electrochemical potentials of desired reactions. An alternate – and less attractive outcome – is charge carrier recombination that generates heat and emission. Our group is modeling photocatalyst heterojunctions composed of metal-free 2D materials as alternatives to titania-based systems.
Effects of Noncovalent Interactions in Functional Supramolecular Assemblies
Noncovalent interactions pervade biological, materials, and chemical systems. Although relatively weak on an individual level, such interactions have a strong impact on molecular structuring and dynamics when acting in concert. The extent that halogen bonding and other noncovalent interactions can direct the assembly of supramolecular structures with specific material characteristics is still being determined. We are developing a new modeling paradigm that provides unique chemical insight regarding the connectivity between the molecular level and larger-scale material properties. It identifies molecular signatures in donors and acceptors that control the assembly of these building blocks into supramolecular structures, thereby advancing our understanding of what dictates the material properties and providing a chemical route to modulate the observed behavior.  For example, an attractive feature of halogen bonding is the intrinsic tunability of interaction strength through modification of the polarizability of the halogen atom, withdrawing capability of the substituents on the halogen-containing molecule, and hybridization of the carbon atom in the C–X bond. The figure shows the effects to the electrostatic potential along the R-X bond upon varying the identity of the halogen atom in halobenzenes. This flexibility, coupled with amphielectronic character, provides an opportunity for cooperativity and orthogonal interactions to generate supramolecular structure with target functionality from molecular-level building blocks.
For example, an attractive feature of halogen bonding is the intrinsic tunability of interaction strength through modification of the polarizability of the halogen atom, withdrawing capability of the substituents on the halogen-containing molecule, and hybridization of the carbon atom in the C–X bond. The figure shows the effects to the electrostatic potential along the R-X bond upon varying the identity of the halogen atom in halobenzenes. This flexibility, coupled with amphielectronic character, provides an opportunity for cooperativity and orthogonal interactions to generate supramolecular structure with target functionality from molecular-level building blocks.
Recent Cover Art
We graciously thank our sponsors for research funding.