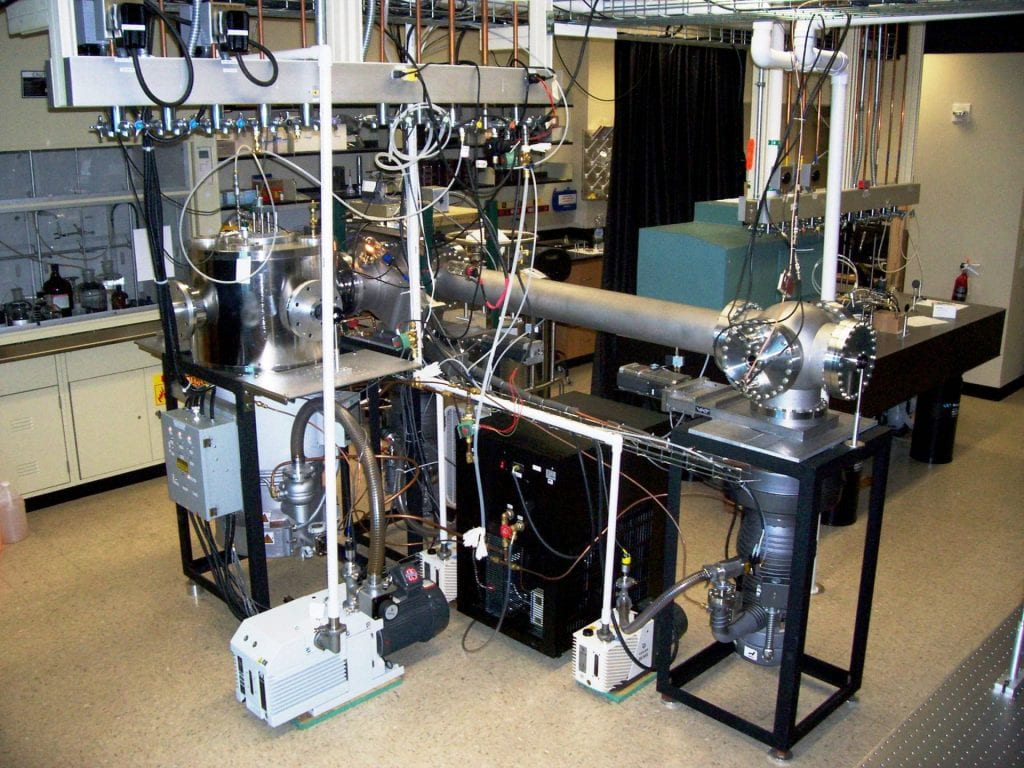
The Bellert Group studies metal ion/molecule reactions to understand chemical reactivity and catalysis. Our research focuses on measuring the energy lowered pathways attributed to the action of transition metal active sites. We are particularly interested in nonadiabatic reaction pathways, such as hydrogen tunneling and two-state reactivity. These represent methods to lower activation energies beyond what is traditionally (adiabatically) possible. Our employed methods are unique in that we do not use a collision to initiate a chemical reaction. Rather, the reactants are formed into a cluster at low temperatures through supersonic expansion. The reaction is initiated by visible laser photon absorption which marks the start-time of the reaction. The temporal dependence of product formation is selectively monitored by time of flight mass spectrometry yielding first-order kinetic signatures. Complete reactions are sampled and we measure the temporal dependence of nuclear motion as bonds are broken and formed. This affords us the ability to identify and characterize nonadiabatic dynamic influences in chemical reactivity.
This laser-based research is unique and required the construction of custom instrumentation. Consequently, we have developed the single photon initiated dissociative rearrangement reaction (SPIDRR) technique to measure metal-mediated chemistry. We have partnered with a theoretical group from Harding University so that together, both the experimental and theoretical characterization of nonadiabatic chemical influences is possible.
The following pages provide greater detail of our experimental technique, research outcomes, hypotheses, recent publications, outreach through instruction, etc.